As our staffers see it, the "one tiny trick"¹ practiced by so many ignorant and semi-ignorant freelancers attempting to answer technical questions is what we like to call the kitchen-sink method. That is taking as many unrelated factoids about the topic, rewording them to avoid being nailed for plagiarism, and calling them done. We're pretty certain that's what happened when Elizabeth Sanker (writing as E. A. Sanker) attempted to explain for some middle-schooler, "What Is Natural Radioactivity?" at the site formerly known as WiseGEEK.com and now moved to a niche site with the ridiculous name InfoBloom.com.²
Just for grins, we decided to ask an expert instead of an English Lit graduate, so we surfed over to the National Institutes of Health website for the following:
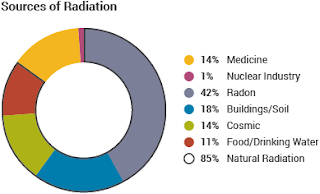
"Natural radiation comprises cosmic radiation and the radiation arising from the decay of naturally occurring radionuclides."
Sanker decided to go the NIH one better, informing her audience that,
"Major sources of natural radioactivity include cosmic radiation, terrestrial radiation, and radiation from material in the human body."
We're still trying to understand how the human body spontaneously generates radioactivity. We would like to go on record as suspecting that any radioactive material in the human body comes from the natural environment either through ingestion or respiration, but maybe that's just us. After all, Sanker minored in biology... Continuing along the factoid compilation, Elizabeth attempted to explain cosmic radiation, telling her readers that,
"Cosmic radiation consists of subatomic particles from outer space, mostly protons and hydrogen nuclei. The sun also emits radiation during solar flares."
Guess she skipped chemistry class the day we learned that a hydrogen nucleus is a proton. What she might have meant to say, but didn't, was "helium nuclei." Oh, and Elizabeth? The sun emits radiation constantly: it's called "light"...
Skipping ahead, we find Sanker trying to explain Carbon-14. While she got the end result of decay mostly right, she failed to understand that 14C is reverting to Nitrogen-14, because the collision of a cosmic ray and an atom of 14N created the 14C atom in the first place! And then she tried to explain radiocarbon dating, telling us that,
"Materials containing carbon-14 can be placed in geological [sic] time using a process known as radiocarbon dating, in which the amount of carbon-14 in the material is used to determine its age."
Both sentences contain misinformation: first, the upper limit of radiocarbon dating is about 50,000 years; a far cry from the 4.5 billion years of geologic time. Second, radiocarbon dating does not use "the amount of carbon-14 in the material," it uses the ratio of 14C to total C. Speaking of radioactive decay, Sanker would have us believe that Uranium decays into radium, which is only true as a half-step: the ultimate daughter element of uranium's decay is lead...
The mot interesting assertion, however, that Sanker made is this:
"...substances in the human body also produce radiation..."
...which reads as though the body is naturally radioactive. That's bullshit. These elements, which Elizabeth ultimately allows are ingested, include "potassium-40, uranium, thorium, radium, and some others [sic]." The most common radioactive element in the body is 40K, but humans also metabolize strontium, which substitutes for calcium in bones and teeth. As for uranium and thorium? Naaaahhhh.
Ultimately Sanker's piece contains about half information and half misinformation, much of the latter a result of attempts to reword what we think was an EPA document (WiseGEEK does not publish references). With a ratio that high, how could we not grant Elizabeth the singular honor of being today's Dumbass of the Day?
¹ Aren't you tired of that stupid come-on? We know are!
² Why isn't it at AllThingsNature.org, by the way? The word "natural" is in the title!
² Why isn't it at AllThingsNature.org, by the way? The word "natural" is in the title!
SI - CHEMISTRY
No comments:
Post a Comment